In May of 2018, researchers announced an exciting new discovery: a drug commonly used to treat osteoporosis – WAY-316606 – may be the answer to millions of people’s hair loss problems (1).
But what does this new study say, and what exactly is WAY-316606 and how might it help hair loss sufferers?
In this post, I’ll introduce you to new compound and its potential benefits. I’ll then break down the current research, as well as:
- How it compares to Cyclosporine A (CsA), a similarly effective drug with devastating side effects;
- Whether there are side effects, and how these may impact your choice;
- Where WAY-3160606 currently is in the FDA approval process, and when you might expect its debut; and
- Why you may want to consider alternative options for treating your hair loss.
Of course, I’ll also share the very methods I used to treat my hair loss, and even reverse it!
What Is WAY-316606?
The drug WAY-316606 is used in the treatment of osteoporosis – a condition that causes fragile bones and increases susceptibility to fractures (2). It’s an SFRP1 inhibitor, which means it prevents the protein SFRP1 from being produced and activated (3).
How Does It Work?
The protein SFRP1 works as a modulator of the Wnt signaling pathways. These pathways – and more specifically the ‘canonical’ pathway – lead to the regulation of gene transcription, which is crucial when determining what role genes will play.
Interestingly, the Wnt signaling pathway has been identified as playing a part in the development of osteoporosis (6). This occurs as the pathway encourages the development of more bone-resorbing cells (osteoclasts) than bone-forming cells (osteoblasts).
It works, then, by triggering the Wnt signaling pathway. This helps the two different cell types to be better differentiated, leading to less bone degradation and further bone growth.
But what does this have to do with hair loss?
While we’ll discuss this more in-depth further down, Wnt signaling pathways also play a role in hair loss.
As hair follicles and their accompanying hairs develop, a process known as cell differentiation takes place. This occurs in the Dermal Papilla (DP), which is essentially the base of the follicle.
During this process, proteins are produced and delivered to various parts of the follicle. However, overexpression of certain proteins – particularly SFRP1 – can lead to quickened cycling and structural defects of the hair strand.
If left uninterrupted, this can lead to follicle miniaturization.

This all takes place as part of the Wnt signaling pathway and is much more common in individuals with hair loss disorders (7).
As WAY regulates the Wnt signaling pathway, it’s believed it can play a role in slowing down protein over-expression and result in regular hair growth and development.
Is It Safer Than Cyclosporine A?
As I’ll discuss at a later point, Cyclosporine A (CsA) is an immunosuppressive drug used to treat psoriasis and even to prevent rejection in organ transplant patients. In clinical trials, it was also shown to have a side effect of hair growth.
So, why isn’t CsA marketed as a hair loss treatment drug?
Because it has numerous serious side effects, including nausea, vomiting, seizures, and muscle and joint pain.
This new drug, on the other hand, does the same job with less severe side effects (if any).
What Does the Research Have to Say?
Through various clinical studies, it was found that the immunosuppressant CsA induced hair growth. However, researchers weren’t entirely sure why. That is, until the 2018 study that was published in PLOS Biology (9).
This study was carried out in various stages, and it revealed three very important things:
- It identified Wnt signaling as a non-immune-inhibitory CsA target;
- It introduced SFRP1 as a regulator of β-catenin activity in a human organ; and
- It demonstrated that WAY-316606 is a promising new drug in the treatment of hair loss.
Let’s take a closer look at how these conclusions were made.
Method and Results
The first step was to determine how CsA works in triggering hair growth. To do so, human hair follicles were treated with CsA ex vivo for six hours. The changes in gene transcription were then measured through microarray analysis.
As results would show, the Wnt inhibitor SFRP1 had the largest decrease after treatment:
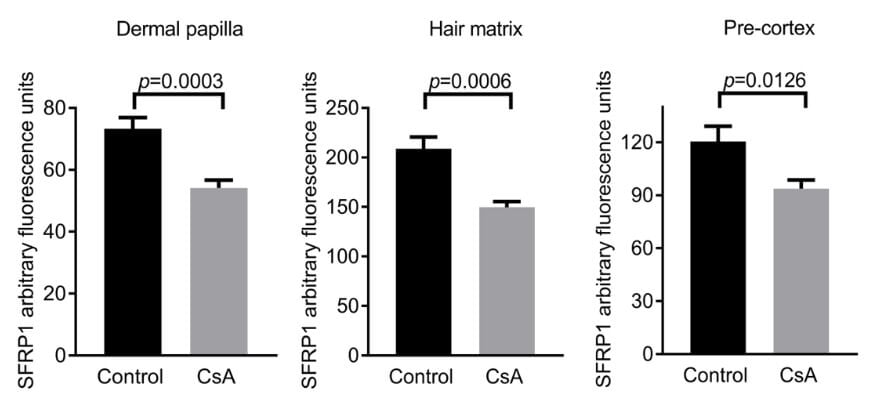
This decrease was marked in the Dermal Papilla (DP), where it was discovered that SFRP1 is produced.
To then confirm these results, human hair follicles were treated with CsA, or a control.
The results showed that the hair follicles treated with CsA were in the anagen phase at a higher rate than the control, both at 4 days and 6 days.
What exactly does it mean as it relates to hair loss?
The presence of SFRP1 induces a premature catagen phase in the hair follicles. When blocked, the hair follicles are in the anagen phase longer, which leads to more growth.
The researchers then asked:
“Does Wnt ligand activity modulate canonical β-catenin in the human HF bulb?”
The Wnt/β-catenin pathway plays a key role in hair follicle stem cell proliferation (10). What researchers wanted to know was whether stimulating this activity via artificial means would stimulate hair growth.
And the answer?
YES!
Human hair follicles were treated with the drug WAY-316606 for 6 days. Throughout, hair shaft production was measured.
WAY-316606 was found to significantly increase hair shaft production, even as early as two days following treatment:

Even better?
It did so much more effectively than even CsA!
WAY-316606 even increased the number of hair follicles in the anagen phase, which is crucial for hair loss management.
Implications
You likely have many questions running through your mind but perhaps most importantly:
“What does this mean for hair loss sufferers?”
The short answer is, yes it could be a promising alternative to the two most popular hair loss treatments available, finasteride and minoxidil. Instead of targeting DHT – which is an important part of male development – it regulates a pathway known to play a part in hair loss.
(Learn more about alternatives to minoxidil here!)
Are There Side Effects?
As previously mentioned, the immunosuppressant CsA has some heavy-duty side effects associated with its use (11). These can range from minor digestive upset, to full-blown kidney failure.
So, you may be wondering:
“Does WAY carry the same risks?”
At this time, it appears not.
CsA is an immunosuppressant that targets multiple pathways. This is believed to be the cause of its toxicity (12).
Alternatively, this new compound is quite selective in the pathways it targets. In fact, 2 μM its shown to inhibit SFRP1 by about 40%, but its relatives (SFRP2 and SFRP5) are only inhibited at 5% and 2%, respectively.
In other words?
WAY targets the cause of early catagen (SFRP1), while leaving other related genes to function as they should.
A Link to Cancer?
While the long-term adverse effects of WAY have yet to be explored, there is one possible risk to consider: cancer.
This has to do with the role that Wnt pathways and, more specifically abberant regulation, plays in cancer development (14).
According to a 2017 review study, the role that Wnt signaling plays in carcinogenesis has been most described in colorectal cancer. However, it has been observed in other cancer types as well.
But how does signaling regulation contribute to cancer? The most likely answer is three-fold: maintenance of cancer stem cells, metastasis, and immune control. In other words, when the signaling pathways are regulated they may trigger a cascade of events which creates the perform storm for cancer development.
That’s not to say that WAY-316606 is sure to cause cancer, especially because the distinction between canonical and non-canonical pathways is still being researched. But the future research studies on the topic will likely address this issue.
So, What Happens Next?
Unfortunately, WAY is not yet available for use and it likely won’t be for years. This is because it must undergo rigorous testing and clinical trials before it can even be considered for human use.
The process may vary, but the vast majority of drugs undergo such a process as outlined below (15).
Step 1: Discovery and Development
This first step is quite direct, in that it involves the discovery (intentional or not) and further development of a pharmaceutical treatment. This is what was done in the May 2018 study mentioned above, and is the first step in every drug development process.
At this stage, researchers may discover drug treatments in a variety of ways. These include:
- As an unintended side effect of any already approved drug on the market.
- As part of new technological advancements.
- As part of a discovery related to a disease and its processes.
Once discovered, the drug must then be thoroughly examined and developed. At this stage, researchers consider how the drug may affect the biological processes, including:
- How it’s absorbed and excreted;
- How it’s delivered throughout the body;
- Its potential mechanisms of action;
- Its potential benefits, and adverse effects;
- How it interacts with other drugs/treatments; and
- Its effectiveness as compared to current drugs/treatments on the market.
Some aspects of this step can bleed over into Step 2, as much research is required before it can be tested more fully on humans.
Step 2: Preclinical Research
At this stage, researchers are beginning to test the drug in the laboratory. This is done in two ways:
- In vitro – These take place in glass or plastic, such as a test tube or petri dish.
- In vivo – These take place within a living organism and, at this stage, that means an animal (such as mice or rats).
The studies performed at this stage are on the small side, but the information collected can then be used to guide further studies. For example, what is the proper dosage for treatment? What are the toxicity concerns? How is the drug best delivered? Etc.
Step 3: Clinical Research
Once researchers have established that a drug is safe for human testing, clinical research begins. There are four main phases of a clinical trial, which are:
- Phase 1: This takes place over several months, and it commonly involves less than 100 participants. The purpose is to determine safety and dosage, which will then be applied to future trials.
- Phase 2: Taking place over several months to two years, the purpose of this trial phase is to determine efficacy and side effects. There are likely to be several hundred participants who suffer from the condition that the drug is believed to treat.
- Phase 3: With anywhere from 300 to 3,000 volunteers with the disease or condition, this phase takes from one to four years. The purpose is to further test efficacy, as well as monitor adverse effects.
- Phase 4: The final phase is the largest one, with several thousand volunteers taking part. Its goal is to establish the drug as safe and effective and, if it passes this phase, will very likely be approved by the FDA.
Whew! That certainly seems like a lot of work. So, is the drug now ready for sale? Not quite!
Step 4: FDA Review
This is the stage that can make or break a drug’s approval, as the FDA must be provided with all information that researchers have collected on the drug. When the application for approval is submitted, the FDA will look closely at:
- Proposed labeling;
- Safety updates;
- Drug abuse information;
- Patient and demographic information; and
- Directions for use.
The FDA will also thoroughly inspect any trials the drug underwent, and heavily consider whether the research backs up its efficacy and safety. The researchers may be required to submit even more information, and they’ll likely collaborate closely with the FDA review board as the review is underway.
Once a drug has passed the approval process, it’s finally available on the market!
Step 5: FDA Post-Market Safety Monitoring
The drug has finally been made available – whether by prescription or over-the-counter. But, the review process still isn’t entirely over.
For as long as the drug is available to consumers, the FDA will monitor and review all drug-related complaints and comments. These may cause them to add warnings to the packaging, to change suggested usage, or to even remove the drug from the market completely.
Where Is It In the Process?
While it has been FDA-approved in the treatment of osteoporosis, the drug will again have to undergo trials and review to determine its use in the treatment of hair loss.
At this stage in the game, it is only at the beginning – discovery and development.
There are still many more years before the drug will be approved as a treatment option, and that’s assuming that it successfully makes it through all stages of the process.
What exactly does this mean for hair loss sufferers?
Sadly, don’t hold your breath. If you wait until this new drug comes out (assuming it even does), your hair loss could be irreversible. Instead, you should focus on other ways to regrow your hair right now.
The Alternative Approach to Hair Loss
Instead of waiting years for the possibility of treatment, why not be proactive and begin fighting hair loss now? By taking the right steps, you can not only stop balding but even reverse your loss.
How?
Take a look at some of the natural methods I’ve personally used!
Scalp Massage and Exercises
As mentioned above, the scalp must receive adequate blood flow. This reduces the chances of follicle miniaturization.
Manual stimulation of the scalp is the best way to boost circulation and improve flow (16). This has been proven in a variety of studies, and scalp massage has even been studied specifically (17).
The two manual methods I recommend? Massage and exercises!
How to Perform Scalp Massage
You can perform scalp massages by yourself, using either your hands or a scalp massaging device.
Here’s how:
- Using your thumbs and middle and index fingers, place each hand on the side of your head (just above your ears). Apply light pressure, and begin working your hands in circular motions.
- While continuing the circular motions (and varying pressure as you go along), move your hands slowly up the sides to the crown of your scalp. You can backtrack to previous locations at any time, and then retrace your steps back to the crown.
- Continue massaging the crown, and surrounding areas. As you do, slowly make your way towards the front of the scalp.
- Let your two hands meet at the middle of your hairline, and then begin to work your way out towards the temples. Do this while continuing the circular motions, and retracing your steps as necessary.
- Finally, return to the sides of your scalp and then to the base. Remain here for 2 minutes, and then you’re all done.
The entirety of this session should take 10 – 15 minutes, and you can practice it every day for improved results.
How to Perform Scalp Exercise
While I favor scalp massages, as they’re quite relaxing, scalp exercises are another way to increase blood flow. They do so by using their facial muscles to exercise often-forgotten areas.
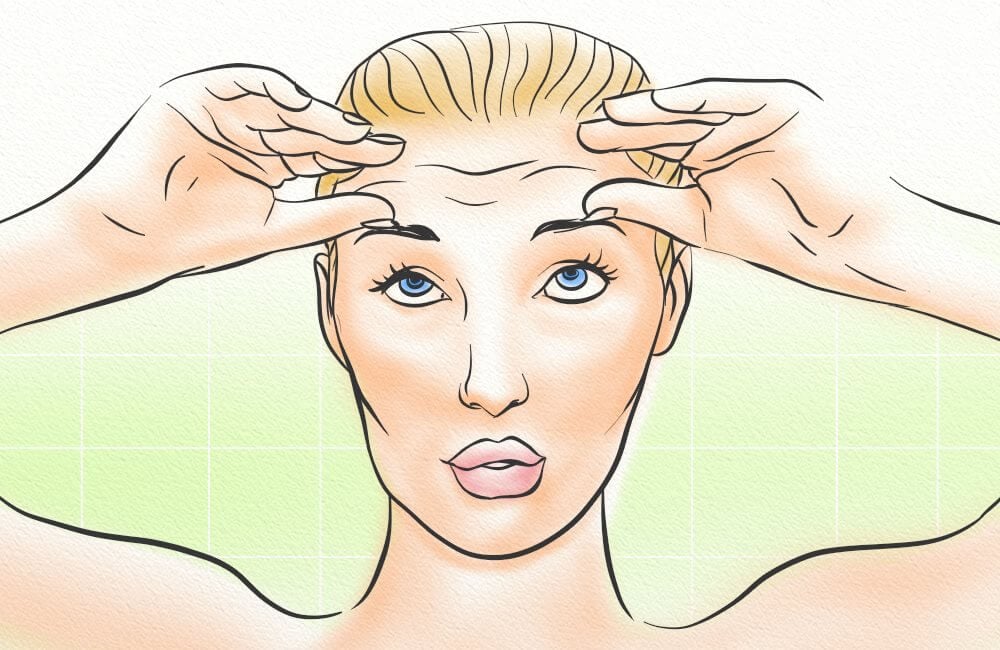
There are three basic steps for a scalp exercise, and they are:
- Lift your eyebrows as high as possible, and hold for 1 – 2 minutes. Return to a relaxed position.
- Furrow your eyebrows as deep as possible, and hold for 1 – 2 minutes. Return to a relaxed position.
- Lift your eyebrows as high as possible, and hold for 1 – 2 minutes. Then furrow your eyebrows as deep as possible, and hold for 1 – 2 minutes. Finally, return to a relaxed position.
You can also use your fingertips for added stretching and smoothing of the muscles and skin.
To do so, place your thumb and middle and index fingers on your scalp in a triangular position. Gently stretch the skin by pulling your fingers away from each other, and then loosen the skin by pushing your fingers closer together.
You can do this all over the scalp but pay particular attention to tight and/or irritated areas.
Microneedling
If you’re looking to further increase blood flow to the scalp, then you’ll want to consider microneedling!
Microneedling is a method that uses tiny needles to create micro wounds. As these wounds heal, new collagen is produced and new cells develop. This lends itself to the production of healthier hair follicles.
But how effective is it really?
Well, the results from a 2013 study speak for themselves (18):

As you can see, the group that used minoxidil alone fared more poorly than the group that used both minoxidil and microneedling. This shows that microneedling can be added to your hair care routine with great success!
How to Use a Dermastamp
There are two main types of microneedling tools – the dermaroller, and the dermastamp.
While I’ve recommended the dermaroller in the past, I’ve actually switched over to the dermastamp more recently (and you should, too!). It’s not only easier to target but there’s also less risk of causing damage or pulling already-present hairs.
You can learn more about how to use the dermaroller here.
Final Thoughts
There’s no doubt that the research released by Dr. Hawkshaw and his team is exciting. In fact, it could play a major role in how hair loss is understood and treated.
But, I urge you to not be blinded by the hype.
You may be waiting for the release of WAY-316606 as a hair loss drug, but this isn’t likely to be for many years (if at all). Instead, I suggest you begin to take steps now to treat your hair loss so as not to suffer any more of the devastating condition.
Information contained on this website has not been evaluated by any medical body such as the Food & Drug Administration. All information is for educational purposes only. We do not aim to diagnose, treat, cure or prevent any disease or illness. You must consult a medical professional before acting on any content on this website.
